What is Chondroitin Sulfate?
Chondroitin sulfate is a natural substance found in the human body and a major component of cartilage, which helps build connective tissue throughout the body, including those that form joints and the gastrointestinal (GI) tract. Because it works by retaining water, it helps add lubrication and flexibility to tissue and joints.
When found in supplement form, it can either be derived naturally from the cartilage of animals (including cows, pigs or sharks) or man-made. Drinking bone broth is probably the greatest way to obtain both glucosamine and chondroitin sulfate at home.
The form of chondroitin made in laboratory settings is called chondroitin sulfate, which is a combination of chondroitin and mineral salts that help improve its absorption. (3) Supplements containing chondroitin can go by many different names depending on the product’s specific formula: chondroitin glucosamine, glucosamine sulfate, and chondroitin sulfate are all names you might encounter, for example. While the terminology might seem confusing, the various forms available can mostly all be used in the same way.
Health Benefits of Chondroitin Sulfate
1. Helps Treat Osteoarthritis Joint Pain
Estimates show that over 27 million adults in the U.S. live with osteoarthritis, which is the most common type of arthritis and degenerative joint disease that’s characterized by the breakdown of cartilage and increased joint pain. Chondroitin sulfate is commonly used to treat pains associated with osteoarthritis, especially forms that affect very susceptible body parts like the knees and hands. Overall, studies have shown that the use of chondroitin tends to cause modest improvements in joint pain over the course of several months, although some people experience even more benefits and more quickly — especially when combining several supplements together and making other changes like eating an arthritis diet to treat symptoms. According to the Arthritis Foundation, on average study participants usually experience about a 10 percent improvement in painful symptoms when using chondroitin compared to placebo. The best results are usually achieved after using a product containing it for three months or more. Recently, the University of Utah’s School of Medicine conducted the largest-ever clinical study investigating the effects of chondroitin and glucosamine, called “The Glucosamine/Chondroitin Arthritis Intervention Trial (GAIT).” According to reports released by the National Center for Complementary and Integrative Health, GAIT is the first large-scale, multicenter clinical trial in the U.S. to test the effects of the dietary supplements glucosamine hydrochloride (glucosamine) and sodium chondroitin sulfate (chondroitin sulfate) for the treatment of knee osteoarthritis.
- The GAIT study compared the effects of glucosamine and chondroitin sulfate (used separately and also in combination) to effects of a placebo and also a prescription drug.
- 16 rheumatology research centers across the U.S. and over 1,500 patients participated in the study, which lasted six months.
- Patients received one of five treatments over the course of six months, including the use of glucosamine and chondroitin, celecoxib (a popular prescription drug used for managing osteoarthritis pain) or a placebo. A positive response to any treatment was defined as a 20 percent or greater reduction in pain after six months compared to the start of the study.
- Results of the GAIT study showed that for participants with moderate to severe pain, glucosamine combined with chondroitin sulfate provided statistically significant pain relief compared with the placebo — about 79 percent had a 20 percent or greater reduction in pain versus about 54 percent for placebo group.
- Chondr0itin and glucosamine actually worked for more people than the prescription did — 70 percent of participants in the celecoxib group experienced pain relief compared to placebo.
However, for participants in the mild pain subset, glucosamine and chondroitin sulfate seemed to do less to reduce their pain. These participants on average didn’t experience statistically significant pain relief like those with more severe pain did.
Results from another randomized, double-blind, placebo-controlled clinical trial that appeared in Arthritis and Rheumatism tested the effects of chondroitin taken by 162 symptomatic patients with osteoarthritis of the hand. The results showed that patients who experienced chronic hand pain and took 800 milligrams of chondroitin sulfate (CS) daily experienced on average modest pain relief, reduced morning stiffness and improvements in overall functionality within three to six months of regular use.
Researchers also found that the majority of patients experienced no adverse side effects due to chondroitin, which often can’t be said of other painkilling medications that can cause adverse effects like stomach ulcers, dependence, digestive issues, blood pressure problems and more. The researchers’ conclusion was that “CS improves hand pain and function in patients with symptomatic OA of the hand and shows a good safety profile.”
2. Helps with Injury and Exercise Recovery
Even for people without osteoarthritis, there’s evidence suggesting that chondroitin used with glucosamine helps preserve valuable cartilage, decreases pain, increases physical function and enhances self-care activities. It can reduce joint stress following exercise or injury by helping the body synthesize new cartilage, keeping joints flexible and controlling the body’s natural inflammatory responses.
3. Improves Wound Healing and Skin Health
Chondroitin and glucosamine are also used together to help heal wounds, skin-related defects, inflammation of the skin and even in veterinary medicine. Chondroitin can help the body produce collagen, which is essential for skin health, healing and fighting the effects of aging on the skin.
Treatments made using chondroitin and glucosamine are often used for wound dressing even for severe wounds, plus applied over scrapes, burns and lesions to keep wounds moist and promote faster recovery. Some studies have even found that in patients with burns requiring skin grafting, the use of chondroitin in treatment gels can speed up healing time and help control inflammation significantly.
Dosage of Chondroitin Sulfate
BY MOUTH:
- For osteoarthritis: the typical dose of chondroitin sulfate is 800-2000 mg taken as a single dose or in two or three divided doses daily for up to 3 years.
APPLIED TO THE SKIN:
- For osteoarthritis: a cream containing 50 mg/gram of chondroitin sulfate, 30 mg/gram of glucosamine sulfate, 140 mg/gram of shark cartilage, and 32 mg/gram of camphor has been used as needed for sore joints for up to 8 weeks.
INJECTED INTO THE MUSCLE:
- For osteoarthritis: chondroitin sulfate (Matrix) has been injected into the muscle daily or twice weekly for 6 months.
INSERTED INTO THE BLADDER:
- For urinary tract infections (UTIs): 50 mL of a specific solution containing chondroitin sulfate and hyaluronic acid (iAluRil, IBSA Farmaceutici), has been inserted into the bladder once weekly for 4 weeks, and then once or twice monthly for up to 5 months.
APPLIED TO THE EYE:
- For cataracts: Several different eye drops containing sodium hyaluronate and chondroitin sulfate (DisCoVisc, Alcon Laboratories; Viscoat, Alcon Laboratories; DuoVisc, Alcon Laboratories; Viscoat, Alcon Laboratories; Provisc, Alcon Laboratories) have been used during cataract surgery.
Potential Side Effects of Chondroitin Sulfate
Chondroitin sulfate is LIKELY SAFE when taken by mouth or used as an eye solution during cataract surgery. Chondroitin sulfate has been taken by mouth safely in research for up to 6 years. Also, chondroitin sulfate has been given premarket approval by the US Food and Drug Administration (FDA) to be used as an eye solution during cataract surgery.
But there is some concern about the safety of chondroitin sulfate because it comes from animal sources. Some people are worried that unsafe manufacturing practices might lead to contamination of chondroitin products with diseased animal tissues, including those that might transmit bovine spongiform encephalopathy (mad cow disease). So far, there are no reports of chondroitin causing disease in humans, and the risk is thought to be low. It can cause some mild stomach pain and nausea. Other side effects that have been reported are bloating, diarrhea, constipation, headache, swollen eyelids, leg swelling, hair loss, skin rash, and irregular heartbeat.
Some chondroitin products contain excess amounts of manganese. Ask your healthcare professional about reliable brands.
Chondroitin sulfate is POSSIBLY SAFE when injected into the muscle short-term, when applied to the skin short-term, when used as an eye drop short-term, and when inserted into the bladder with a catheter by a physician.
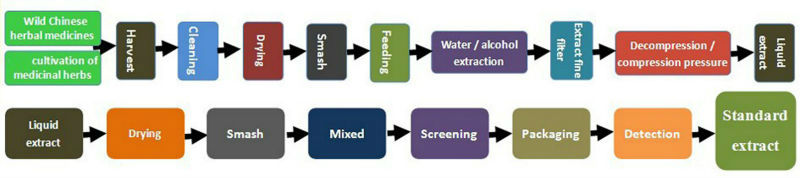
Flowchart of Production
Package and Shipping

- By Express: Suitable for under 50kg, 5-10 days
- By Air: Suitable for more than 50kg, 7-15 days
- By Sea: Suitable for more than 500kg, 15-45days
Why Choose FYZ Chondroitin Sulfate?
- FYZ is a professional Chondroitin Sulfate manufacturer in China, provides private labels service.
- FREE SAMPLE (5-10g) for detection, if you need more, please contact us.
- Fast delivery by DHL/FedEx, air as your requirement.
- All of our products from nature, no additive.
- Money refund policy.







