What Is Andrographis Paniculata Extract Andrographolide?
Andrographis paniculata is an annual herbaceous plant in the family Acanthaceae, native to India and Sri Lanka. It is widely cultivated in Southern and Southeastern Asia, where it has been traditionally used to treat infections and some diseases. Mostly the leaves and roots were used for medicinal purposes. The whole plant is also used in some cases.
Components of Andrographis Paniculata Extract
Andrographolide is the major constituent extracted from the leaves of the plant and is a bicyclic diterpenoid lactone. This bitter principle was isolated in pure form by Gorter (1911). Systematic studies on chemistry of A. paniculata have been carried out.
Some known constituents are:
- “14-Deoxy-11-dehydroandrographolide, Plant
- 14-Deoxy-11-oxoandrographolide, Plant
- 5-Hydroxy-7,8,2′,3′-Tetramethoxyflavone, Plant
- 5-Hydroxy-7,8,2′-Trimethoxyflavone, Tissue Culture
- Andrographine, Root
- Andrographolide, Plant
- Neoandrographolide, Plant
- Panicoline, Root
- Paniculide-A, Plant
- Paniculide-B, Plant
- Paniculide-C, Plant”
Health Benefits of Andrographis Paniculata Extract Andrographolide
1. Andrographis Paniculata Extract And Antimicrobial
Extracts of Andrographis and andrographolide derivatives have shown modest activity in vitro against HIV; however, a phase 1 study of andrographolide showed no effect on viral replication after 6 weeks, despite increased CD4 counts.
Activity in antimalarial screens has also been noted for Andrographis extracts, but clinical trials are lacking.
The extract of A. paniculata blocked Escherichia coli enterotoxin-induced secretion in rabbit and guinea pig models of diarrhea. Andrographolide and 3 other related diterpenes were responsible for this action. Other in vitro experiments present conflicting results for the action of andrographolide and leaf extracts on E. coli.
2. Cancer
Animal and in vitro experiments using human cancer cell lines to investigate the potential anticancer effects of A. paniculata have found andrographolide responsible for the observed effects rather than other diterpenes. Various mechanisms of action have been proposed, including enhancement of chemokine activity, inhibition of tumor-specific angiogenesis affecting cell cycle progression, and induction of apoptosis. Cancer cell lines investigated include prostate, breast, cervical, colon, hepatoma, melanoma, and lymphocytic leukemia. Researchers are now focusing on synthesizing compounds based on andrographolide to improve selectivity and potency.
The need for caution has been raised by one group of researchers because andrographolide-enhanced SDF-1-chemokine activity might induce tumor cell metastasis. A. paniculata extract has also induced cell differentiation in mouse myeloid leukemia cells.
3. Immunostimulant
Animal data
Both antigen-specific and antigen-nonspecific immune responses in mice were stimulated by andrographolide and an ethanolic extract; the extract was more potent than andrographolide, suggesting that other constituents also were immunostimulants. Inhibition of passive cutaneous anaphylaxis and mast cell stabilization was observed in studies of the purified diterpenes in rats.
Clinical data
A systematic review of 4 clinical trials found A. paniculata , either alone or in a fixed combination with A. senticosus ( Kan Jang ), significantly more effective than placebo ( P < 0.0001 and P = 0.0002, respectively) in reducing the severity of upper respiratory tract infections and related symptoms. The review found a lack of outcome consistency in the included studies. Other clinical trials have demonstrated similar results for respiratory infections, but methodology in these trials is of poor quality.
A small study in patients with familial Mediterranean fever (N = 24) found a decrease in frequency, duration, and severity of febrile attacks, compared with placebo.
Stimulation of the production of key cytokines and immune activation markers has been investigated as potential mechanisms of andrographolide immunomodulation.
4. Other uses
Extracts of Andrographis have demonstrated hypoglycemic action in rats with streptozotocin- and alloxan-induced diabetes, supporting a traditional use of Kalmegh. Clinical trials are lacking.
Two older trials explored a possible hypotensive effect of andrographolide/Kalmegh, but further investigation has not been conducted.
Animal experiments suggest that the extract of A. paniculata is hepatoprotective, but clinical trials are lacking. Hepatic drug-metabolizing enzymes were elevated in 1 animal experiment, however, in a clinical trial, elevated liver enzymes (AST and ALT) were reported.
Andrographolide has demonstrated anti-inflammatory effects in several cellular systems, including prevention of phorbol ester-induced reactive oxygen species and N-formyl-methionyl-leucyl-phenylalanine-induced adhesion in rat neutrophils, inhibition of tumor necrosis factor-induced upregulation of intercellular adhesion molecule expression, and monocyte adhesion and activation of protein kinase pathways.
Dosages of Andrographis Paniculata Extract Andrographolide
Kalmegh dosage in clinical studies has ranged from 3 to 6 g of the crude plant.
The usual daily dose of andrographolides for common cold, sinusitis, and tonsillitis is 60 mg, but doses up to 1,200 mg have been reported. Andrographolide 48 mg daily was used in a trial of familial Mediterranean fever. Doses of 5 to 10 mg/kg were used in a trial in HIV patients, but adverse reactions at this dosage stopped the trial.
Clinical trials in children with upper respiratory tract infection reported the use of andrographolide 30 mg daily for 10 days.
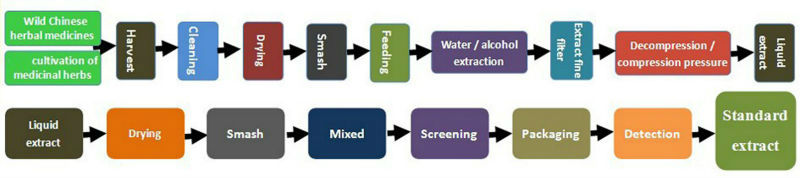
Flowchart of Production
Package and Shipping

- By Express: Suitable for under 50kg, 5-10 days
- By Air: Suitable for more than 50kg, 7-15 days
- By Sea: Suitable for more than 500kg, 15-45days
Why Choose FYZ Andrographis Paniculata Extract Andrographolide?
- FYZ is a professional Andrographis Paniculata Extract Andrographolide manufacturer in China, provides private labels service.
- FREE SAMPLE (5-10g) for detection, if you need more, please contact us.
- Fast delivery by DHL/FedEx, air as your requirement.
- All of our products from nature, no additive.
- Money refund policy.







